Gas Lantern
Background
A gas lantern is a lightweight, portable device that supplies bright, efficient light while protecting its contents from wind and rain. Rural dwellers and outdoorsmen alike have relied on variations of the modern gas lantern for roughly 100 years, allowing access to barns, cabins, campgrounds, and wooded paths beyond the daylight hours.
This style of lantern is more practical than its ancestors because it operates on the principle of incandescence—rather, it relies on light produced by heat. The heated mantles in a gas lantern emit far more light than the flame of an oil lamp, therefore providing better visibility in a larger area. Mantles are chemically saturated fabric shells that, when heated by the lantern's flame, become a powerful source of white light—up to 300 candlepower, or the rough equivalent of a 300-watt bulb.
History
For an untold number of years, the open flame was humankind's only source of controlled light. Early ceramic lamps dating back to the Roman era were little more than earthenware pots with tubes to supply vegetable oil to a wick and spout. Centuries of development attempted to master the potential of lamplight, employing variations of fuel and wick materials to boost efficiency, but it was not until the nineteenth century that scientists and inventors began to make vast improvements in light quality.
By the 1830s, a portable lamp had been developed using a pressure mechanism to force fuel oil to the burner. This concept, paired with the arrival of the first durable working mantle in 1885, led to the modern styles of portable lanterns used during the last hundred years.
Austrian chemist Carl Auer von Welsbach is credited with the invention of the modern thorium mantle. Through his work with rare earth metals, Auer von Welsbach discovered that certain oxides would give off incandescent light when heated. Original Welsbach mantles came in the form of loosely woven silk fabric impregnated with magnesium and lanthanum oxides. Six years later, he had settled on a mix consisting of 99% thorium, a silvery-white metal with a melting point of almost 6,000°F (3,300°C). This ability to withstand immense heat allowed it to emit higher levels of brilliant white light. Historians note that Auer von Welsbach's progress in this area was partially driven by a sense of developmental urgency; his work was in direct competition with that of the incandescent electric light.
It would be decades before reliable electric service would reach outside of urban communities, and the demand for usable light swelled in rural homes and workplaces. A forerunner to the modern lantern was known as the Efficient Lamp, manufactured by the Connecticut-based Edward Miller Company. The portable Efficient Lamp used a pressure system to vaporize gasoline, mix it with air, and ignite it in a burner to heat the mantles. In 1900, part-time typewriter salesman W. C. Coleman happened across an Efficient Lamp in the window of an Alabama drugstore. Fascinated by the lamp's intensity, Coleman sought out its owners and immediately began selling the product himself. Two years later, he bought the rights to the design, made some improvements, and renamed it the Coleman Arc Lamp. Over the next decade, variations of pressure mantle lamps emerged from Coleman and several competitors, including the Western Lighting Company (now Aladdin), whose founder was similarly inspired by a German kerosene mantle burner called the "Practicus."
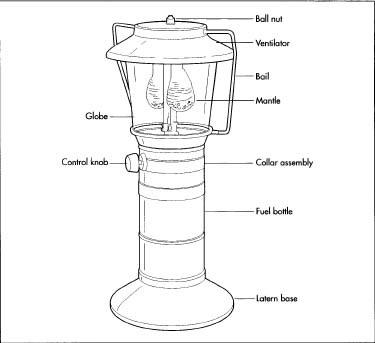
The Coleman Arc Lantern, introduced in 1914, was the first in a long succession of portable gas lantern models. Able to illuminate a circle 100 ft (30 m) in diameter, the Arc Lantern featured a protective metal hood to ward off wind, rain, and curious insects. Its bail (handle) and stout shape allowed the Arc Lantern to be easily carried, hung from a branch or rafter, or set to the ground.
Revisions of the Arc lantern were to remain in production for the next 53 years. Initial improvements in the 1920s introduced the "Instant-Lite Lanterns," which eliminated the need to preheat the generator. In earlier models, the generator would have to be manually heated before it would vaporize fuel; this involved holding a match or a burning piece of felt (usually fuel-soaked) up against it. Later innovations brought multi-fuel lanterns that would burn kerosene, gasoline, benzine, petrol, or paraffin. Advances in metallurgy following World War II led to non-corrosive steel founts, or fuel tanks. The development and use of heat-resistant glass also solved a key design issue: that a hot glass globe tended to shatter when hit with cold rain.
Enhancements over the years have made the traditional lantern brighter, lighter to carry, and simpler to use. Newer electric-start models no longer require a match. Propane bottle fuel now eliminates the need for building pressure manually. However, even considering these alterations, the straightforward design of a portable lantern has remained essentially unchanged since the early decades of this century.
Raw Materials
High-grade steels comprise the majority of a lantern's components. The ventilator hood and fount are usually draw-quality, meaning that the steel is flexible and will not crack under the pressure of a deep press. Various brass alloys are used to make parts of the fuel delivery system; the grade used for each part depends on how much heat that particular piece needs to withstand. Other steel alloys are used for smaller parts such as the bail, collar, and pressure and ignition systems. The standing base and control knobs in more recent models have been made of molded plastic or rubber.
While some globes are made of a metal mesh, heat-resistant borosilicate glass is still the most prevalent material used in globe production. Often sold under the brand name Pyrex, the glass is formed from a combination of silica sand and boric oxide.
Mantles consist of a silk or rayon mesh saturated with various chemicals. Thorium is still commonly used but often criticized—applications for the slightly radioactive thorium include the manufacture of nuclear weapons. In response to safety concerns, makers in the United States now substitute the pricier but non-radioactive element yttrium, which gives off a more yellowish tone.
Design
Modern designs are tailored to different needs. Though the standard, durable lantern of past decades still enjoys a devoted market, design engineers now consider convenience, utility, and even cosmetic concerns in the development of new models. For serious campers and climbers, a class of small, lightweight lanterns is available; the light output is minimal, but consumer concern in this case is for portability. For standard uses, however, design competitors experiment with higher grades of steel, better fuel efficiency, and a hardier shell. Features like metal cages around the globe, self-gauging pressure pumps, electric ignitions, and non-slip rubber bases are becoming aspects of a new production standard for gas lanterns. The mantles themselves have also been subject to improvements in shape, material, and size.
The Manufacturing
Process
Making steel components
-
1 To form molten steel, iron ore is melted with coke, a carbon-rich
substance that results when coal is heated in a vacuum. Depending on the
alloy, other metals such as aluminum, manganese, titanium, and zirconium
may also be introduced. After the steel cools, it is formed into sheets
between high-pressure rollers and distributed to the manufacturing
plant.
 A double mantle propane lantern.
A double mantle propane lantern. - 2 There, metal presses shape the steel into the appropriate parts. This process is not entirely mechanized, however; multi-step manual operations are required to move the steel from press to press.
Enameling the steel
- 3 This is usually done via "e-dip," a water-based process used to give lanterns their signature colors. The steel components are cleaned and manually set on a large conveyor. These parts then receive an electric charge, which determines the thickness of paint when dipped and ensures an even coating.
- 4 After primer, paint, and topcoat dips, the parts are then bake-dried. However, because e-dipping is expensive, smaller components are often enameled by an automated paint sprayer. This is a process in which static electricity attracts paint to the object, minimizing overspray and airborne toxins. A significant amount of hand labor is involved in this method, which requires parts to be hung on hooks before enameling.
Making the plastic parts
- 5 Small plastic pieces such as knobs and buttons are often made by outside vendors. To form these objects, plastic pellets are added to the hopper of an injection molding machine. The plastic is melted, and a hydraulic screw pushes the substance through a nozzle, where it is injected into a pre-shaped mold, held under pressure, and cooled. Factory staff transport the finished parts, but the process is otherwise fully automated.
Making the globes
- 6 Globe production involves a multi-cavity horizontal wheel, usually with six molds. Hot borosilicate glass is pushed in tube form off a feeder nozzle and onto the wheel. A layer of compressed air is then blown against the molds and the wheel is spun, forming the globe shapes. The glass edges are fired automatically, and the glass is left to cool.
Making the mantles
- 7 Silk or synthetic string is shipped to the factory by vendors, with the rest of production done in-house. The delicacy of mantles requires that the "sock" be made by hand with the help of sewing machinery, with some automated conveyor systems employed to move the process along more efficiently.
- 8 Factory personnel then hang the unfinished mantles in preparation for an automated chemical dip. Chemical impregnation processes vary and are usually considered trade secrets by mantle manufacturers.
Assembly
- 9 Before the lantern is fully assembled on the main conveyor lines, a process called sub-assembly gathers the smaller parts and connects them into larger systems. Main assembly involves a "square line," a four-sided conveyor manned by three or four personnel. Pre-assembled parts, such as the fuel and pressure systems, are screwed to the fount. Down the line, workers use nuts and screws to complete the final assembly phase, which involves mounting the collar and attaching the globe, ventilator, and bail.
Quality Control
The feature that buyers consistently look for in a lantern is durability. These products are expected to last, trouble-free, for decades. Because of these standards, visual and mechanical inspection is necessary at every step. During the design process, in-house quality assurance teams brainstorm and troubleshoot in an effort to form individual specifications for each product. This includes the required grade levels of materials, inspection protocol, and machinery pressure and temperature management. Manufacturers must also adhere to governmental regulation; these standards include those related to occupational safety, emissions, and the transport and packaging of products containing potentially volatile fuels.
Byproducts/Waste
No byproducts result from the manufacture of gas lanterns. Waste is minimal due to the fact that most of the production materials can be reused. The yttrium used in mantles, since it is fairly expensive, is conserved and recycled for the purpose of efficiency. Metal alloys are recycled as much as possible, but scraps do comprise one example of industrial leftovers. The only examples of hazardous waste are called VOCs (volatile organic compounds), which are formed in the enameling process. However, the technologies used in this stage are designed to keep VOC levels to a minimum and as far below government limits as possible.
The Future
When new technologies become available, research and development teams present these options to the engineering and design staff, who then decide whether to incorporate them into a product. Gas lanterns, however, are less susceptible to drastic change because of their simple design. Although lanterns using alternative light sources are widely sold, employing battery, electric, and solar power, the rustic and utilitarian appeal of a gas lantern will likely keep the product from undergoing any major system overhauls. Nevertheless, new possibilities for materials and ease of operation are always a significant priority.
Where to Learn More
Books
Hobson, Anthony. Lanterns that Lit Our World, Book Two. New York: Golden Hill Press, 1997.
Other
"A Brief History of the Incandescent Mantle Pressure Lamp." Pressure Lamps Unlimited Web Page. 1998. December 2001. < http://ourworld.compuserve.com/homepages/awm/history.htm >.
Coleman Company, Inc. A Brief History of the Use of Coleman Lamps and Lanterns. Pamphlet, 1980.
"Dr. Carl Auer von Welsbach: Portrait." Auer-von-Welsbach Museum Web Page. December 2001. < http:llwww.althofen.at/welsbach.htm >.
"For a Better Lantern—Borax." Corning Museum of Glass Web Page. December 2001. < http:llwww.cmog.org >.
Oral interview with Richard Long, Senior Engineer at Coleman Company, Wichita, KS. December 2001.
Kate Kretschmann
Comment about this article, ask questions, or add new information about this topic: