Oxygen Tank
Background
Oxygen (atomic number, 8; atomic weight, 16) is essential for all living things and has the ability to combine with almost all other elements. When elements fuse with oxygen, they are labeled as being oxidized. Oxygen is the most plentiful element in the world, comprising about 90% of water (hydrogen makes up the other 10%) and 46% of the earth's crust (silicon, 28%; aluminum, 8%; and iron, 5%; among others). Oxygen's melting point is −360°F (−218°C) and its boiling point is −297°F (−183°C). In its free state, oxygen is odorless, colorless, and tasteless. At temperatures below −297°F (183°C) oxygen takes on a pale blue liquid form.
Two-thirds of the human body is composed of oxygen. In humans oxygen is taken in through the lungs and distributed via the blood stream to cells. In the cells, oxygen combines with other chemicals, making them oxidized. The oxidized cells are then distributed where they are needed, providing the body with energy. The waste products of respiration are water and carbon dioxide, which are removed through the lungs.
Pressurized oxygen therapy is used to treat numerous medical aliments such as emphysema, asthma, and pneumonia. This medicinal form of oxygen is typically kept in medium-sized aluminum canisters equipped with pressure regulators and release valves. Large amounts of oxygen are kept in large, insulated steel tanks pressurized at 2,000 lb/in 2 (141 kg/cm 2 ).
History
The discovery of oxygen has generally been attributed to Joseph Priestley, an English chemist. In 1767, Priestly believed that air mixed with carbon was able to produce electricity. He called this carbonized air, mephitic air. Priestly went on to conduct experiments concerning air, and in 1774 he used a burning glass and solar heat to heat mercuric oxide. While doing this, he noticed that the mercuric oxide broke down under the extreme temperature and formed beads of elemental mercury. The mercuric oxide also emitted a strange gas that facilitated flames and opened the respiratory tract, making it easier to breath when inhaled. This gas was named dephlogisticated air by Priestley, based on the popular thought of the time that phlogiston was needed for material to burn. The phlogiston theory was deemed false by Antoine-Laurent Lavoisier, a French chemist.
Lavoisier had been conducting his own experiments with combustion and air in the mid- to late-eighteenth century. It was in 1774, that he met Priestley who told Lavoisier of the discovery of dephlogisticated air. Lavoisier began to conduct his own experiments on Priestley's pure form of air. He observed that the element was part of several acids and made the assumption that it was needed to form all acids. Based on this incorrect thought, Lavoisier used the Greek words oxy (acid) and gene (forming) to coin the French word oxygene—translated to oxygen in English—sometime around 1779.
There is yet a third man who is credited for his involvement in the discovery of oxygen in about 1771. Carl Wilhelm Scheele, a Swedish pharmacist and chemist, discovered that a certain element (Scheele also thought it to be phlogiston) was needed in order for substances to burn. Scheele called this element "fire air" due to it being needed for combustion. During these experiments with fire air, Scheele also discovered "foul air," now known as nitrogen. Despite the fact that Scheele had isolated oxygen before Priestley, Priestley published his findings first.
Raw Materials
The raw materials to produce an oxygen tank are liquid air and aluminum. The aluminum starting stock is cast 6061. The liquid air is condensed and heated until pure oxygen remains then distributed into the aluminum tanks. A compressible Teflon ring is used to form the o-ring, which is placed in the o-gland forming a seal between the valve and the cylinder. The o-ring gland is a precision depression machined in the top of the cylinder. When the valve is screwed in the cylinder and when completely seated, it compresses the o-ring and completes the airtight seal between the valve and the cylinder.
Design
Oxygen tanks vary in size, weight, and function but the manufacturing process is very similar. The typical medicinal oxygen tank contains pure oxygen and has a green top with a brushed steel body.
The Manufacturing
Process
Formation of the cylinder
- 1 Oxygen tanks are manufactured from a single sheet of 6061 aluminum. The starting material is called a cast billet, which is approximately 18 ft (5.5 m) long and shaped like a log.
- 2 The cast billet is placed on a conveyor belt and cut to the desired size by an automated saw. The sawn piece is called a slug and is almost the same weight and diameter as the finished product.
- 3 The slug is then placed inside a die in a backward extrusion press. The press forces a punch against the slug. The metal of the slug flows backwards around the punch forming a large, hollow, cup-shaped product called a shell.
- 4 The shell is then inspected for defects and gauged.
- 5 Next, the shell is put through a process called swaging. The open end of the shell is heated and forced into a closing die to close the open end of the cup. Now the general shape of a seamless cylinder is finished.
Heat treating the cylinder
- 6 The cylinder is conveyed through a two step thermal process called solution heat treat and artificial aging.
- 7 The first thermal process, solution heat treatment, begins when the cylinder is placed in a solution furnace. In this process the alloying elements of the aluminum are put into the solution. The cylinder is heated to about 1,000°F (538°C). A cylinder that has been subjected to this thermal process is labeled as being in the T-4 temper.
- 8 The second thermal cycle, artificial aging, consists of the cylinder being conveyed through an age oven where it is heated to about 350°F (177°C). This allows the alloying elements to precipitate out of the solution and into the grain boundaries, strengthening the cylinder. A cylinder that has completed both thermal processes is labeled as being in the T-6 temper.
The neck configuration
- 9 The threads, o-ring gland, and top surface are the sealing surfaces and are machined into the cylinder. The cylinder is placed in a milling machine (a drill press able to move in three directions). Under the direction of Computer-Aided Design (AutoCAD) software, a hole is milled in the center of the cylinder's neck.
- 10 The top surface, o-ring gland, and threads (in that order) are machined into the cylinder using a form tool. The form tool has the form of the top of the cylinder, the o-ring gland and the thread relief are under the o-ring. The form tool spins as a drill bit and is lowered into the cylinder machining the form into the cylinder neck.
Finishing
-
11 The tank is then subjected to hydrostatic testing. During this test
the tank is pressurized equal to five-thirds its service pressure. If
the tank expands greater than a
specified amount within 30 seconds, it is rejected.
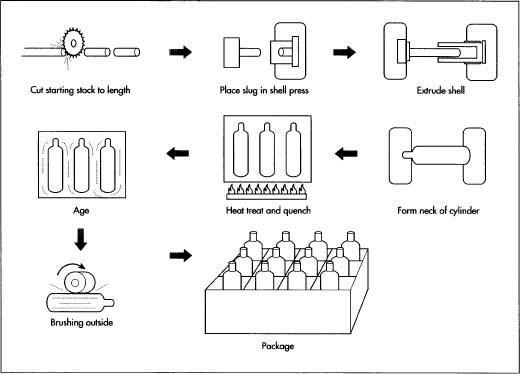 The manufacturing of oxygen tanks.
The manufacturing of oxygen tanks. - 12 Identification marks are stamped onto the tank via a pneumatic stamper. These marks identify the specifications to which the cylinder was manufactured, service pressure, serial number, manufacturer's name or number, and the manufacturing date of the tank.
- 13 The tanks used for medical purposes generally have a brushed body. The tank is placed horizontally on the conveyor belt and rotated under an automatic sander.
- 14 The top of the tank is manually painted green then the entire tank is sprayed with a clear powder coat and cured in an oven.
- 15 The finished tank is then either capped or equipped with a valve depending on the customer requirements.
Filling the tanks
- Commercial pressurized oxygen is distilled from liquid air in large batches. Air becomes liquid at −297°F (−183°C). The air supply is compressed, then passed through a compartment equipped with a piston (expansion engines).
- As the air expands, the pistons move, increasing the volume of the compartment and decreasing the pressure and temperature of the air.
- The air is then rotated through several expansion engines until liquefied. The liquid air is then transported to huge insulated holding tanks.
- The liquid oxygen is then boiled to get rid of the nitrogen, since nitrogen has a lower boiling point (−320'F; 195°C). The liquid air is then mostly oxygen (97-100%) and transported to large insulated tanks until dispersed into oxygen cylinders.
Quality Control
During the manufacturing process, the cylinders are inspected and cleaned numerous times. After the tank is sold and put into service, it must be put through hydrostatic and visual retesting every five years. The testing is conducted in accordance to the Compressed Gas Associations requirements. If the tank is not damaged and wear is minimal, there is unlimited service life.
DOT-3AL is the marking identifying the specification in which the cylinder was manufactured in compliance. The Department of Transportation (DOT) regulates the transportation of all goods. The transportation of compressed gases falls into this category.
Byproducts/Waste
In the manufacturing process nearly 93% of the starting material (the cast billet) is used in the final product. There is less than 7% manufacturing scrap of the starting material. After production is complete, any cylinders that are damaged to the point of being condemned are stamped through the "DOT-3AL" marking on the crown. If the tank has been pressurized, it is depressurized, the valve is removed, and the cylinder is sawn in half and recycled. The condemned, sawn cylinders can and should be recycled.
The Future
As the medical use of oxygen tanks increases, the tanks are getting smaller and more maneuverable. The standard medical E tank holds 680 l and can provide up to 11.3 hours at 1 liter per minute (lpm). This tank weighs 7.9 lb (3.6 kg) empty. One of the smaller oxygen tanks is an M9 tank. This tank holds 240 l of oxygen lasting for four hours at 1 lpm or two hours of continuous flow. There are accessories such as carts or bags that allow the user to transport the full tank easily.
Where to Learn More
Other
Catalina Cylinders Web Page. 8 November 2001. < http://www.catalinacylinders.com >.
Tri-Med, Inc. Web Page. 8 November 2001.< http://www.trimed.freeservers.com >.
Deirdre S. Blanchfield
Comment about this article, ask questions, or add new information about this topic: