Silver
Background
Silver was one of the earliest metals known to humans, and it has been considered a precious metal since ancient times. Silver has been used as a form of currency by more people throughout history than any other metal, even gold. Although it is usually found in ores with less rare metals, such as copper, lead, and zinc, silver was apparently discovered in nugget form, called native silver, about 4000 B.C. Silver utensils and ornaments have been found in ancient tombs of Chaldea, Mesopotamia, Egypt, China, Persia, and Greece. In more recent times, the principal uses for silver were coinage and silverware.
In 1993, worldwide production of silver from mines totaled 548.2 million ounces (15.5 billion grams). During that year, Mexico was the world's largest producer of silver, with a total production of 75.7 million ounces (2.1 billion grams). The United States was the second leading producer, followed by Canada, Australia, Spain, Peru, and Russia. The vast majority of the world's silver is used in industrial applications, and the United States is the leading consumer. Other top consumers include Japan, India, and eastern European countries.
Silver mining in North America dates back to the eighteenth century. Around 1800, production began in the United States on the east coast and then moved west. The mining of silver was instrumental in the settlement of the state of Nevada. In 1994, Nevada was the largest producer of silver in the United States; Nevada mines produced 22.8 million troy ounces (709 million grams) of silver. Arizona, California, and Nevada are known for large-tonnage, low-grade silver deposits.
Physical Characteristics and Uses of Silver
Silver is the whitest metallic element. It is rare, strong, corrosion resistant, and unaffected by moisture, vegetable acids, or alkalis. Silver is also resonant, moldable, malleable, and possesses the highest thermal and electric conductivity of any substance. The chemical symbol for silver is Ag, from the Latin argentum, which means white and shining. Although silver does not react to many chemicals, it does react with sulfur, which is always present in the air, even in trace amounts. The reaction causes silver to tarnish, therefore, it must be polished periodically to retain its luster.
Silver possesses many special physical characteristics and qualities that make it useful in a variety of industries. The photography industry is the biggest user of silver compounds. Silver forms the most light-sensitive salts, or halides, which are essential to developing high-quality photography. Silver has the highest electrical conductivity per unit volume of any metal, including copper, so it is used extensively in electronics. Specialized uses include switch and relay contacts for automobile controls and accessories, automotive window heating, and in electrodes for electrocardiograms.
Silver is one of the strongest oxidants, making it an essential catalyst for the chemical process industry. It is used in the production of adhesives, dinnerware, mylar recording tape, and many other products. Silver is the most reflective of all metals,
Today, a very small percentage of the world's silver is used in coinage, though silver coins were a popular form of currency until the recent past. As industrialized nations began to produce large numbers of silver coins in the twentieth century, silver became less available, and therefore more expensive. The United States Treasury, which until then had been minting 90% silver coins, changed their minting by a 1965 act of Congress. The Johnson Silver Coinage Act completely demonetized silver, and with the exception of bicentennial coins, all newly-minted United States coins are now made of an alloy of copper and nickel.
The Manufacturing
Process
Silver was first obtained in sixteenth-century Mexico by a method called the patio process. It involved mixing silver ore, salt, copper sulphide, and water. The resultant silver chloride was then picked up by adding mercury. This inefficient method was superseded by the von Patera process. In this process, ore was heated with rock salt, producing silver chloride, which was leached out with sodium hyposulfite. Today, there are several processes used to extract silver from ores.
A method called the cyanide, or heap leach, process has gained acceptance within the mining industry because it is a low-cost way of processing lower-grade silver ores. However, the ores used in this method must have certain characteristics: the silver particles must be small; the silver must react with cyanide solutions; the silver ores must be relatively free of other mineral contaminants and/or foreign substances that might interfere with the cyanidation process; and the silver must be free from sulfide minerals. The idea for cyanidation actually dates back to the eighteenth century, when Spanish miners percolated acid solutions through large heaps of copper oxide ore. The process developed into its present form during the late nineteenth century. The cyanide process is described here.
Preparing the ore
-
1 Silver ore is crushed into pieces, usually with 1-1.5 in (2.5-3.75 cm)
diameters, to make the material porous. Approximately 3-5 lb (1.4-2.3
kg) of lime per ton of silver ore is added to create an alkaline
environment.
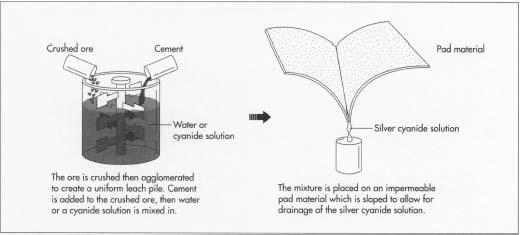
The ore must be completely oxidized so the precious metal is not confined in sulfide minerals. Where fines or clays exist, the ore is agglomerated to create a uniform leach pile. This process consists of crushing the ore, adding cement, mixing, adding water or a cyanide solution, and curing in dry air for 24-48 hours.
- 2 Broken or crushed ore is stacked on impermeable pads to eliminate the loss of the silver cyanide solution. Pad material may be asphalt, plastic, rubber sheeting, and/or clays. These pads are sloped in two directions to facilitate drainage and the collection of the solutions.
Adding the cyanide solution and curing
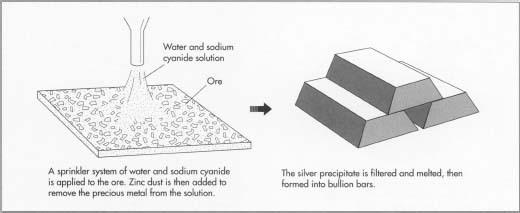
- 3 A solution of water and sodium cyanide is added to the ore. Solutions are delivered to the heaps by sprinkler systems or methods of ponding, including ditches, injection, or seepage from capillaries.
Recovering the silver
- 4 Silver is recovered from heap leach solutions in one of several ways. Most common is Merrill-Crowe precipitation, which uses fine zinc dust to precipitate the precious metal from the solution. The silver precipitate is then filtered off, melted, and made into bullion bars.
- 5 Other methods of recovery are activated carbon absorption, where solutions are pumped through tanks or towers containing activated carbon, and the addition of a sodium sulfide solution, which forms a silver precipitate. In another method, the solution is passed through charged resin materials which attract the silver. The recovery method is generally decided based on economic factors.
Silver is rarely found alone, but mostly in ores which also contain lead, copper, gold, and other metals which may be commercially valuable. Silver emerges as a byproduct of processing these metals. To recover silver from zinc-bearing ores, the Parkes process is used. In this method, the ore is heated until it becomes molten. As the mixture of metals is allowed to cool, a crust of zinc and silver forms on the surface. The crust is removed, and the metals undergo a distillation process to remove the zinc from the silver.
To extract silver from copper-containing ores, an electrolytic refining process is used. The ore is placed in an electrolytic cell, which contains a positive electrode, or anode, and a negative electrode, or cathode, in an electrolyte solution. When electricity is passed through the solution, silver, with other metals, accumulates as a slime at the anode while copper is deposited on the cathode. The slimes are collected, then roasted, leached, and smelted to remove impurities. The metals are formed into blocks which are used as anodes in another round of electrolysis. As electricity is sent through a solution of silver nitrate, pure silver is deposited onto the cathode.
The Future
How much silver will be produced in the future depends on many factors, including the rate of production of other metals and future uses of silver. Industrial demand for silver appears to be steady overall. Because silver naturally occurs with other metals, future production is linked to the production of copper, lead, gold, and zinc.
In the future, silver will likely continue to be used for special industrial applications, as well as for consumer items, such as jewelry and silverware. In addition to these traditional uses, the value of silver will also depend on new uses for the metal. For example, using silver as a sanitizing agent is currently under development. Manufacturers have hustled in response to studies by the Atlanta-based Center for Disease Control that many viruses, including those linked to Acquired Immunodeficiency Syndrome (AIDS), will survive briefly outside an individual in fluids deposited on surfaces of plastic products, such as telephones. Matsushita Electric Industrial Co., Ltd. in Osaka, Japan, completed a project at the Research Institute for Microbial Diseases, Osaka University, to produce a surface treatment that provides long-lasting sanitization for its plastic products. Research revealed the most effective system to be a compound based on silver thiosulfate.
Currently marketed under the name Amenitop, the system consists of silica gel microspheres that contain a silver thiosulfate complex. The silica gel coating allows a gradual release of the silver compound onto the surface, which provides long-lasting sanitization. Studies suggest that Amenitop kills bacteria and viruses by destroying the cell's membranes.
Where to Learn More
Books
Coombs, Charles. Gold and Other Precious Metals. William Morrow and Company, 1981.
Robbins, Peter and Douglass Lee. Guide to Precious Metals and Their Markets. Nichols Publishing, 1979.
Smith, Jerome F. and Barbara Kelly Smith. What's Behind the New Boom In Silver and How to Maximize Your Profits. Griffin Publishing Company, 1983.
Periodicals
"A Listing of the End Users of Silver By Property." The Silver Institute, January 13, 1994, pp. 1-14.
"A Nevada Leader." News from Las Vegas (Las Vegas News Bureau), March 1995, p. 1.
"New Silver Compound To Fight Spread of Viruses." The Silver Institute Letter, December 1994-January 1995, pp. 1, 2,4.
Thorstad, Linda E. "How Heap Leaching Changed The West." World Investment News, February 1987, pp. 31, 33.
— Susan Bard Hall