Litmus Paper
Litmus paper is the most recognized member of chemical indicators. Like most pH paper, litmus changes color when exposed to an acidic or basic solution. The simple pH scale ranges from 0-14 with 0 being the most acidic, 7 being neutral, and 14 being the most basic or alkaline. Litmus paper is commonly used in educational science classes. Because it has such wide recognition, it has become a cultural reference in our society as well. It is common to use the term litmus test when referring to a test in which a single factor determines the outcome.
Background
Litmus paper allows an observer the opportunity to assess a sample's pH. pH is a way to characterize the relative acidic or basic nature of a substance based on its hydrogen ion concentration. An ion is an atom that carries an electrical charge and is therefore reactive with its environment. An acidic substance releases hydrogen ions (H+) in water. Acids are known as proton donors because the H+ ion has one extra positively charged proton trying to stabilize itself by combining with a negatively charged ion. A basic substance releases a hydroxide ion (OH-) in water. Bases are called proton acceptors because the hydroxide ion will accept a proton to stabilize itself. Interestingly enough, when acids and bases are combined, the result is a neutral salt. For example, a strong acid like hydrochloric acid combined with sodium hydroxide (a strong base) results in a neutralization reaction with the byproducts sodium chloride (table salt) and water.
pH is an important biological indicator because most life forms have a very small range of pH in which they can survive. For example, the acid-base ratio in the human body is a delicate balance. Even a slight change in the blood's pH in either direction can result in death. Plants are also susceptible to minute pH changes in the soil. That is why soil that is too acidic for a plant is neutralized with calcium carbonate fertilizer, a base.
The simple pH scale ranges from 0-14 with 7 being neutral. Numbers less than 7 are considered to be acidic and numbers greater than 7 are considered basic. The smaller the number the more acidic the solution. This means that a substance with a pH of 1 would have a greater ability to donate a proton to another molecule or ion than a substance with a pH of 4. For instance, sulfuric acid is very effective at transferring a hydroxide ion, while acetic acid (vinegar) is not. Therefore, sulfuric acid is considered to be a strong acid and acetic acid is considered a weak acid. Similarly, there are also strong and weak bases. A strong base like potassium hydroxide, with its more abundant hydroxide ions, will more readily accept protons than a weak base like ammonia. The greater the number, the stronger the base.
While litmus paper is effective at indicating whether a substance is acidic or basic, it cannot report an exact numerical pH value. Universal indicators or pH meters are used for this purpose. Universal indicators are composed of a variety of materials, each changing different colors at different pH values which allows the observer to determine more precisely where the solution in question falls on the pH scale. Universal indicators can be impregnated onto paper and made into pH paper or they can be used in the liquid form. A reference color card is provided with each universal indicator that correlates a particular color with a pH range. Generally speaking, most universal indicators are accurate to within two values on the pH scale. For example, a green result could indicate a pH from 8-9. This means universal indicators can determine the pH of a sample quantitatively within a certain range.
pH meters allow for even more precise quantification by using electricity to determine a numerical pH value. A probe is put in the test sample and a current of electricity flows through the probe. Since electricity is composed of electrons, which have a negative charge, the force of current flowing through the meter is directly proportional to the hydrogen ion concentration. The more H+ ions in the solution, the more current will flow through the meter. This number is then converted into a numerical pH value that can be read by the observer.
History
The term litmus comes from an Old Norse word meaning "to dye or color." This is fitting since the lichens used to make litmus have also been used to dye cloth for hundreds of years. Very little information is available about the beginnings of litmus. There is some data that suggest that litmus paper was developed by J.L. Gay-Lussac, a French chemist during the early 1800s. Gay-Lussac is best known for his Law of Combining Volumes, which states that whenever gases are formed or react with one another at a constant temperature and pressure, their volumes are in small whole number ratios. In other words, when gases combine, they always do so in the same way provided that the temperature and pressure stays the same.
Raw Materials
The primary raw materials used for making litmus paper are wood cellulose, lichens, and adjunct compounds. Litmus paper, as its name implies, is primarily composed of paper. The paper used to make litmus paper must be free of contaminants that could change the pH of the system it is measuring. Like most paper, litmus paper is made from wood cellulose. The wood is treated with solvents prior to paper manufacturing in order to remove resinous material and lignin from the wood. One of the most common solvents in the United States is a sulfate—either sodium sulfate or magnesium sulfate.
The ability of litmus paper to change color when exposed to an acid or base is a result of litmus paper being infused with lichens. In the plant world, lichens are unique in that they are actually two distinct organisms, a fungus and an alga, living as one. Botanists classify lichens as fungi because it is the fungi that are considered to be responsible for sexual reproduction. However, each lichen has its own distinct name. Approximately 15,000 different types of lichens have been identified. Lichens can be found growing on rocks, trees, and walls, in the soil and even under water in virtually all types of climates. Lichens are commonly used as gauge for environmental quality because they are sensitive to various pollutants. Several varieties of lichen are used to produce litmus including rocella tinctoria, native to the Mediterranean, and lecanora tartarea, a common lichen in the Netherlands. In fact, the Netherlands is one of the largest producers of litmus paper products.
Design
Most litmus paper and other types of pH indicators are sold through scientific supply houses. Litmus paper is available in both red and blue varieties. The natural color for litmus paper is blue. When put in an acidic solution the blue paper turns red. Red litmus paper is first mixed with an acid when it is made. This causes the paper to appear red. When put in the presence of a base, the paper returns to its natural blue color.
The Manufacturing Process
The production of litmus paper has many features in common with paper manufacturing. In this process, the wood pulp is converted to paper, the paper is infused with the lichen solution, and the paper is dried and packaged.
Converting wood pulp
-
1 In this first step, wood is shredded and mixed with a solvent and
water under steam pressure. The resulting mass is called wood pulp. The
pulp is spread on a belt of wire mesh and passed over rollers. This
creates
a thin layer that can be dried and made into usable paper. A wooden box beneath the belt catches water runoff. Since there is salvageable fiber in this water, it is re-mixed with the pulp.
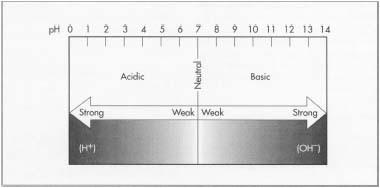 The pH scale.
The pH scale. - 2 To speed up the drying process, air suction pumps beneath the belt are used. As the paper moves along the belt it passes under a mesh or wire cylinder called a dandy roll. The purpose of the dandy roll is to give the paper a weave or watermark that identifies the paper grade and the manufacturer. The paper continues on its journey down the conveyor belt where it is pressed between two felt-covered rolls (couching rolls) that force the fibers to bind together by expressing out additional water.
- 3 From these couching rolls the paper is pushed through two sets of smooth metal press rolls leaving the paper with a smooth finish. The paper is completely dried by subjecting, it to heated rollers, cut by revolving cutters and wound onto reels.
Infusion of lichens
- 4 To make the paper pH active, it is then infused with an aqueous solution consisting mostly of lichens. This is done by running the paper through a bath of the solution. It absorbs the solution and is then passed to allow it to ferment and dry.
- 5 The lichens are allowed to ferment in the presence of potassium carbonate and ammonia. After fermentation, the mass has a blue color and is then mixed with chalk. Blue litmus paper is prepared by impregnating white paper in an infusion of the litmus mixture mentioned above. The paper is then carefully dried in open air. Red litmus is similarly prepared but a small percentage of sulfuric or hydrochloric acid is added to cause it to turn red.
Packaging
- 6 After the paper is prepared, it is sent to a final packaging station. Litmus paper is typically sold in pre-cut strips. Manufacturers place the strips in re-sealable plastic vials. It is important that the packaging prevents the strips from becoming exposed to moisture since any liquid that comes into contact with the litmus paper could cause the indicator to change color. It is possible, although not as common, to purchase litmus paper in rolls that can be cut by the user. Manufacturers also supply written directions with every package of litmus paper so that the user will know how to use the product correctly. In the case of universal indicator papers or solutions, written instructions are not enough. A color reference card is also supplied so the user can match the test result to the reference card to determine the pH.
The Future
Litmus paper will most certainly continue to be used extensively in education due to its reasonable cost and ease of use. However, some varieties of lichens are becoming extinct. As a result, it is possible that manufacturers of litmus paper may switch to synthetic materials in the future. This is already being done by manufacturers of other types of pH papers. Additionally, because litmus cannot give quantitative results, it cannot replace other pH papers and pH meters. In fact, the trend is to make pH indicators that are even more accurate and less subjective. One such trend is to utilize fiber optic probes in pH meters in order to make them even more sensitive.
Where to Learn More
Books
Brady, George S. Materials Handbook. 14th Edition. New York: McGraw Hill, 1997.
Daub, William G., and William S. Seese. Basic Chemistry. 7th Edition. Upper Saddle, NJ: Prentice Hall, 1996.
LaRoe, Edward T. Our Living Resources. Washington, DC: U.S. Department of Interior-National Biological Service, 1995.
"Lichen." In Van Nostrand's Scientific Encyclopedia. 8th Edition. New York: Douglas M. Considine, 1995.
Other
Botanical.com . http://www.botanical.com (January 2001).
Hanna Instruments Online. http://www.hannainst.com (January 2001).
Kiwi Web Chemistry and New Zealand. http://www.chemistry.co.nz (January 2001).
Precision Labs. 9889 Crescent Park Drive Westchester, OH. (513) 777-3034.
— Sandy Delisle and
Perry Romanowski