Fluoride Treatment
Background
A fluoride treatment is a mineral solution applied to teeth in order to strengthen them and help prevent cavities. Fluoride containing products include commercially available toothpaste and mouth rinse, as well as more concentrated liquids and gels used professionally by dentists.
There are three primary factors that contribute to dental caries (tooth decay): a susceptible site on a tooth, an infective strain of bacteria (Streptococcus mutans), and sugars or other nutrients that stimulate the bacteria's growth. As these bacteria grow, they produce an acidic byproduct that can dissolve the minerals in the enamel and eventually destroy the tooth.
Dentists largely credit the use of fluoride treatments and fluoridated water with the drastic decline in tooth decay over the past several decades. One report indicates that for children ages 5-7 years old in the United States the average incidence of cavities has dropped from 7.1% in the 1970s to 2.5% in the 1990s. Similar improvements have been documented across almost all age groups. Still, tooth decay remains the most common infectious childhood disease and fluoride treatments remain an important tool in the fight against cavities.
Fluoride is actually a form of the element fluorine. In its elemental form fluorine is a toxic gas, but when it is chemically reacted with other compounds, such as tin, it takes on new cavity fighting uses. Once in the mouth, fluoride is diluted in saliva and deposited in bacterial plaque on the surface of the teeth. Here it works to protect the tooth in two ways. First, it directly inhibits bacterial growth so less acid is produced in the mouth. Second, the fluoride stored in plaque is released when the bacteria produce enough acid to lower the acid-base balance on the tooth. When this occurs fluoride diffuses into the tooth through tiny pores in the enamel. Fluoride ions replace the hydroxyl ions of the hydroxyapatite crystals, which are part of the tooth's enamel, and form a new compound called fluorapatite. This form of enamel is less soluble in the acids produced by oral bacteria and therefore helps protect teeth from decay.
History
Frederick S. McKay, a dentist practicing in Colorado Springs, Colorado in the early 1900s, was the first to discover that fluoride is an effective cavity fighter. McKay noticed that many of his patients had mottled enamel, or brown stains, on their teeth. By 1916, McKay and his researchers had found that the mottling was caused by something in the patients' drinking water. It took him 12 more years to understand how this effect was related to caries, and another three years to recognize the chemical mechanism causing this change. Finally, in 1931 in McKay verified that patients with mottled teeth were drinking water with unusually high levels of naturally occurring fluoride. This connection was studied in more detail throughout the 1930s and 1940s, culminating in the determination that one part per million was the ideal level of fluoride in drinking water, substantially reducing decay while not causing mottling.
This research led to the implementation of a fluoridation program by the federal government, and by the early 1950s most United States communities with a public water sys-tem had adopted fluoride treated water. The idea of using fluoride in oral care products began in 1956 when Procter and Gamble launched "Crest with Fluoristan." Since the 1950s, scores of fluoride containing products have been introduced both for the general market and for dental professionals.
Even though fluoride has been used for decades, there are still concerns about its health effects today. Although the chemistry is not fully understood, researchers believe that high levels of fluoride can interrupt the natural formation of tooth enamel. They theorize that too much fluoride creates a hypomineralization, which leads to the chalky, cloudy, or opaque appearance that is characteristic of fluorosis. While dental proponents claim that fluoride is largely responsible for improved dental health, there are those who claim that it can cause a form of bone cancer. In the 1980s a study conducted by the National Toxicology Program found "equivocal evidence" of carcinogenicity based on testing done on rats. However, the panel eventually concluded that there was no solid data linking cancer, including osteosarcoma, directly to fluoridation.
Both United States and British Dental Associations continue to recommend that both adults and children brush twice daily with fluoride toothpaste, but they also recommend that to reduce the risk of fluorosis, children should not swallow the toothpaste. The subject remains a hot topic of political debate.
Raw Materials
There are a variety of fluoride compounds that are allowed by the Food and Drug Administration (FDA) for use in oral care products. Fluoride ingredients are listed according to the type of product in which they may be used and by the percentage that must be included in the formula.
Toothpaste. Sodium fluoride: 0.22%; sodium monofluorophosphate: 0.76%; stannous fluoride: 0.4%.
Treatment rinse. (The pH, or acid-base balance, of the formula can affect the functionality of the fluoride. The higher the pH of the product the more acid it contains. The lower the pH, the more basic it is.) Sodium fluoride acidulated with a mixture of sodium phosphate, monobasic, and phosphoric acid to a level of 0.1 molar phosphate ion and a pH of 3.0—4.5, which yields an effective fluoride ion concentration of 0.02%; sodium fluoride acidulated with a mixture of sodium phosphate, diabasic, and phosphoric acid to a pH of 3.5, which yields an effective fluoride ion concentration of 0.01%; sodium fluoride 0.02% aqueous solution with a pH of approximately 7; sodium fluoride 0.05% aqueous solution with a pH of approximately 7; sodium fluoride concentrate containing adequate directions for mixing with water before using to result in a 0.02% or 0.05% aqueous solution with a pH of approximately 7; stannous fluoride concentrate marketed in a stable form and containing adequate directions for mixing with water immediately before using to result in a 0.1% aqueous solution.
Treatment gel. Stannous fluoride: 0.4%.
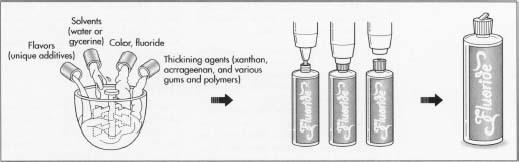
In addition to fluoride, these products contain a variety of other ingredients including solvents, thickeners, and pH control agents. Solvents include water or glycerine, which are used as a carrier for reasons of efficacy, safety, and cost. Deionized or demineralized water is used to prevent unwanted minerals from affecting the performance or stability of the product. The concentration of water in the formula may be 90% or more.
Thickening agents are added to control viscosity. These include xanthan, carrageenan, and various other gums and polymers used at concentrations between 0.1-2.0%.
Flavors and colors are added to make the products more appealing. Popular flavors include mint, bubble gum, and grape, and these are added at a few tenths of a percent. Dyes are used to impart color at very low levels (less than a hundredth of a percent). Since the product may be swallowed accidentally these dyes must be approved for use in food products.
Preservatives are added when necessary. Depending on the pH of the product, they may be required to prevent the growth of mold or bacteria in the product while it is stored the shelf. One or two tenths of a percent is a typical use level for a preservative.
Organic acids, such as phosphoric acid, may be added to control the product's pH. Some
Design
Fluoride treatments are designed to provide an appropriate concentration of fluoride at a pH level that will help the correct amount of fluoride deposit on the teeth. If the fluoride level is too low the treatment will not be effective; if it is too high the patients might accidentally be poisoned. In the United States, laws have been created to ensure that these products are safe and efficacious. The FDA regulates them as either over-the-counter (OTC) drugs or as professional products for use by dentists. In addition, the FDA limits the size of commercially available products to reduce the possibility of accidental overdose. Finally, the organization determines labeling requirements for all commercial products and some aspects of professional ones. These requirements most be taken into consideration when designing fluoride treatments.
OTC fluoride-containing drugs include toothpaste and mouth rinse. Professional products are more concentrated and may be applied either as a gel, foam, or liquid. They may be designed to be applied using plastic trays that fit around the teeth. Depending on the type of product being formulated, the development chemist can choose from several types of FDA approved actives. Once again, these regulatory factors must be considered in product design.
Key examples of treatment formulations are: acidulated phosphate fluoride gel with 1.23% fluoride ion at pH 3.5, designed to flow easily during tray placement yet thickens during treatment so it does not drip down the patient's throat; sodium fluoride gel with 2% fluoride ion at pH 7.0, for use when etching of porcelain restorations is a concern; stannous fluoride liquid rinse with 0.63% fluoride ion, designed to prevent decay, reduce plaque accumulation, and help reduce gingival inflammation and bleeding; APF fluoride foam with 1.23% fluoride ion at pH 3-4.25, designed as an easy to use non-aerosol foam that reduces accidental ingestion by the patient.
The Manufacturing
Process
Batching
- 1 The process of manufacturing a fluoride containing treatment is similar to the processes used to make other oral care products. Because most of the fluoride compounds used in treatments are water-soluble, these products are relatively easy to make. The manufacturing process involves simple mixing and does not require any special solvents or emulsification. Large batches can be made in stainless steel tanks as large as 3,000 gal (11,356 1). The first step is to charge the batch tank with water or glycerine, which makes up the largest percentage of the formula. The water is stirred with an electrically driven turbine mixer. The mixing speed is computer controlled to optimize the stirring conditions when other ingredients are added. While manufacturing procedures vary widely, it is common to add the color early in the batching process. The dyes that are used in these products are highly concentrated and if too much dye is added by mistake it is easier and cheaper to dispose of the batch before the more expensive ingredients have been added.
- 2 The other ingredients may be added in succession. Depending on the solubility of the form of fluoride chosen, heating and cooling may be required to help dissolve the powders quickly. During this batching process samples may be taken periodically during the mixing process to check for clarity. Toward the end of the batching process the pH control agents are introduced. They ensure the batch has the proper acid base balance. Flavors are added at the end of the operation if they are heat sensitive.
Batch check
- 3 Once the batch is complete, it must be evaluated to ensure it is within specification. It is particularly important to ensure that the active ingredients are present at their designated concentrations. Tests such as pH analysis, weight percentage of solids, and fluoride concentration are used to maintain product specifications.
Filling operations
- 4 Once the batch is finished and approved, the filling operation can proceed. Depending on the nature of the process, the batch may be filled directly from the batch tank or it may be transferred to a secondary vessel or holding tank. High speed filling equipment is used but the filling speed depends on the product's viscosity. Thin, liquid products can be filled faster and more efficiently than thicker gels.
- 5 The filled package is fed down an assembly line where the closure is attached. While a bottle may only require a simple cap to seal it, products packed in tubes or foaming dispensers may involve more complicated sealing mechanisms. After the package has been filled and closure has been attached, the unit is ready for final packing. Multiple units may be shrink wrapped together or placed in cartons for shipping.
Quality Control
In addition to the chemical tests conducted during the manufacturing process, fluoride treatments are subject to special testing considerations to establish product performance. Historically these tests have involved expensive human clinical studies, but since 1988 the Dental Panel has allowed the use of new laboratory tests to determine the efficiency of fluoride treatments.
The Future
While the political future of fluoride treatments may be uncertain, they continue to be an important tool in the fight against cavities. There are new technological advances that may some day lead to fluoride free cavity fighters. British researchers have discovered a new kind of anti-caries agent that stops tooth decay for up to three months. Their new ingredient is a protein fragment, called peptide p1025 that works by attaching itself to the tooth surfaces where cavity-causing bacteria normally bind. The protein blocks the bacteria from attaching to teeth so they are easily washed away. Breakthroughs like this could someday provide fluoride-free ways to prevent tooth decay.
Where to Learn More
Books
Wolinsky, L. E. "Caries and Cariology." In Oral Microbiology and Immunology. 2nd ed. Ed. R. J. Nisengard and M. G. Newman. Philadelphia: W. B. Saunders Company, 1994.
Periodicals
Brady, Robert P., and Abbe Goldstein. "Keeping Faith in Fluoride." Chemist & Druggist (24 May 1997): 24.
"Mouthwash Cancels Cavities." Popular Mechanics (February 2000): 15.
"Postmenopausal Osteoporosis Treatment with Fluoride." American Family Physician (January 1996): 302.
Sheikh, Aamir, and Alice M. Horowitz. "Benefits of Fluoride Toothpaste." Journal of School Health (October 1999): 299.
Other
Connelly. "Caries Treatment with Fluoride." United States Patent 5738113, 1998.
Randy Schueller