Insulin
Background
Insulin is a hormone that regulates the amount of glucose (sugar) in the blood and is required for the body to function normally. Insulin is produced by cells in the pancreas, called the islets of Langerhans. These cells continuously release a small amount of insulin into the body, but they release surges of the hormone in response to a rise in the blood glucose level.
Certain cells in the body change the food ingested into energy, or blood glucose, that cells can use. Every time a person eats, the blood glucose rises. Raised blood glucose triggers the cells in the islets of Langerhans to release the necessary amount of insulin. Insulin allows the blood glucose to be transported from the blood into the cells. Cells have an outer wall, called a membrane, that controls what enters and exits the cell. Researchers do not yet know exactly how insulin works, but they do know insulin binds to receptors on the cell's membrane. This activates a set of transport molecules so that glucose and proteins can enter the cell. The cells can then use the glucose as energy to carry out its functions. Once transported into the cell, the blood glucose level is returned to normal within hours.
Without insulin, the blood glucose builds up in the blood and the cells are starved of their energy source. Some of the symptoms that may occur include fatigue, constant infections, blurred eye sight, numbness, tingling in the hands or legs, increased thirst, and slowed healing of bruises or cuts. The cells will begin to use fat, the energy source stored for emergencies. When this happens for too long a time the body produces ketones, chemicals produced by the liver. Ketones can poison and kill cells if they build up in the body over an extended period of time. This can lead to serious illness and coma.
People who do not produce the necessary amount of insulin have diabetes. There are two general types of diabetes. The most severe type, known as Type I or juvenile-onset diabetes, is when the body does not produce any insulin. Type I diabetics usually inject themselves with different types of insulin three to four times daily. Dosage is taken based on the person's blood glucose reading, taken from a glucose meter. Type II diabetics produce some insulin, but it is either not enough or their cells do not respond normally to insulin. This usually occurs in obese or middle aged and older people. Type II diabetics do not necessarily need to take insulin, but they may inject insulin once or twice a day.
There are four main types of insulin manufactured based upon how soon the insulin starts working, when it peaks, and how long it lasts in the body. According to the American Diabetes Association, rapid-acting insulin reaches the blood within 15 minutes, peaks at 30-90 minutes, and may last five hours. Short-acting insulin reaches the blood within 30 minutes, it peaks about two to four hours later and stays in the blood for four to eight hours. Intermediate-acting insulin reaches the blood two to six hours after injection, peaks four to 14 hours later, and can last in the blood for 14-20 hours. And long-acting insulin takes six to 14 hours to start working, it has a small peak soon after, and stays in the blood for 20-24 hours. Diabetics each have different responses to and needs for insulin so there is no one type that works best for everyone. Some insulin is sold with two of the types mixed together in one bottle.
History
If the body does not produce any or enough insulin, people need to take a manufactured version of it. The major use of producing insulin is for diabetics who do not make enough or any insulin naturally.
Before researchers discovered how to produce insulin, people who suffered from Type I diabetes had no chance for a healthy life. Then in 1921, Canadian scientists Frederick G. Banting and Charles H. Best successfully purified insulin from a dog's pancreas. Over the years scientists made continual improvements in producing insulin. In 1936, researchers found a way to make insulin with a slower release in the blood. They added a protein found in fish sperm, protamine, which the body breaks down slowly. One injection lasted 36 hours. Another breakthrough came in 1950 when researchers produced a type of insulin that acted slightly faster and does not remain in the bloodstream as long. In the 1970s, researchers began to try and produce an insulin that more mimicked how the body's natural insulin worked: releasing a small amount of insulin all day with surges occurring at mealtimes.
Researchers continued to improve insulin but the basic production method remained the same for decades. Insulin was extracted from the pancreas of cattle and pigs and purified. The chemical structure of insulin in these animals is only slightly different than human insulin, which is why it functions so well in the human body. (Although some people had negative immune system or allergic reactions.) Then in the early 1980s biotechnology revolutionized insulin synthesis. Researchers had already decoded the chemical structure of insulin in the mid1950s. They soon determined the exact location of the insulin gene at the top of chromosome 11. By 1977, a research team had spliced a rat insulin gene into a bacterium that then produced insulin.

In 1891, Frederick Banting was born in Alliston, Ontario. He graduated in 1916 from the University of Toronto medical school. After Medical Corps service in World War I, Banting became interested in diabetes and studied the disease at the University of Western Ontario.
In 1919, Moses Barron, a researcher at the University of Minnesota, showed blockage of the duct connecting the two major parts of the pancreas caused shriveling of a second cell type, the acinar. Banting believed that by tying off the pancreatic duct to destroy the acinar cells, he could preserve the hormone and extract it from islet cells. Banting proposed this to the head of the University of Toronto's Physiology Department, John Macleod. Macleod rejected Banting's proposal, but supplied laboratory space, 10 dogs, and a medical student, Charles Best
Begining in May 1921, Banting and Best tied off pancreatic ducts in dogs so the acinar cells would atrophy, then removed the pancreases to extract fluid from islet cells. Meanwhile, they removed pancreases from other dogs to cause diabetes, then injected the islet cell fluid. In January 1922, 14 year-old Leonard Thompson became the first human to be successfully treat-ed for diabetes using insulin.
Best received his medical degree in 1925. Banting insisted Best also be credited, and almost turned down his Nobel Prize because Best was not included. Best became head of the University of Toronto's physiology department in 1929 and director of the university's Banting and Best Department of Medical Research after Banting's death in 1941.
In the 1980s, researchers used genetic engineering to manufacture a human insulin. In 1982, the Eli Lilly Corporation produced a human insulin that became the first approved genetically engineered pharmaceutical product. Without needing to depend on animals, researchers could produce genetically engineered insulin in unlimited supplies. It also did not contain any of the animal contaminants. Using human insulin also took away any concerns about transferring any potential animal diseases into the insulin. While companies still sell a small amount of insulin produced from animals—mostly porcine—from the 1980s onwards, insulin users increasingly moved to a form of human insulin created through recombinant DNA technology. According to the Eli Lilly Corporation, in 2001 95% of insulin users in most parts of the world take some form of human insulin. Some companies have stopped producing animal insulin completely. Companies are focusing on synthesizing human insulin and insulin analogs, a modification of the insulin molecule in some way.
Raw Materials
Human insulin is grown in the lab inside common bacteria. Escherichia coli is by far the most widely used type of bacterium, but yeast is also used.
Researchers need the human protein that produces insulin. Manufacturers get this through an amino-acid sequencing machine that synthesizes the DNA. Manufacturers know the exact order of insulin's amino acids (the nitrogen-based molecules that line up to make up proteins). There are 20 common amino acids. Manufacturers input insulin's amino acids, and the sequencing machine connects the amino acids together. Also necessary to synthesize insulin are large tanks to grow the bacteria, and nutrients are needed for the bacteria to grow. Several instruments are necessary to separate and purify the DNA such as a centrifuge, along with various chromatography and x-ray crystallography instruments.
The Manufacturing
Process
Synthesizing human insulin is a multi-step biochemical process that depends on basic recombinant DNA techniques and an understanding of the insulin gene. DNA carries the instructions for how the body works and one small segment of the DNA, the insulin gene, codes for the protein insulin. Manufacturers manipulate the biological precursor to insulin so that it grows inside simple bacteria. While manufacturers each have their own variations, there are two basic methods to manufacture human insulin.
Working with human insulin
- 1 The insulin gene is a protein consisting of two separate chains of amino acids, an A above a B chain, that are held together with bonds. Amino acids are the basic units that build all proteins. The insulin A chain consists of 21 amino acids and the B chain has 30.
- 2 Before becoming an active insulin protein, insulin is first produced as preproinsulin. This is one single long protein chain with the A and B chains not yet separated, a section in the middle linking the chains together and a signal sequence at one end telling the protein when to start secreting outside the cell. After preproinsulin, the chain evolves into proinsulin, still a single chain but without the signaling sequence. Then comes the active protein insulin, the protein without the section linking the A and B chains. At each step, the protein needs specific enzymes (proteins that carry out chemical reactions) to produce the next form of insulin.
STARTING WITH A AND B
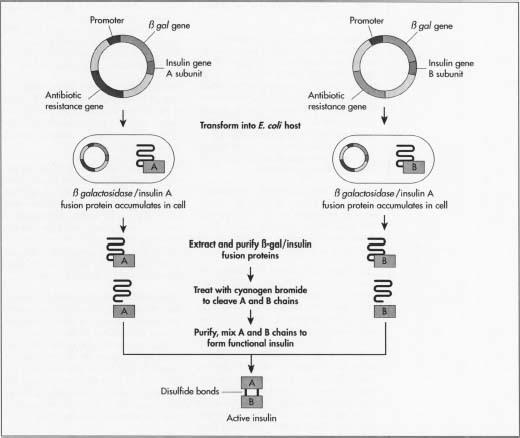
- 3 One method of manufacturing insulin is to grow the two insulin chains separately. This will avoid manufacturing each of the specific enzymes needed. Manufacturers need the two mini-genes: one that produces the A chain and one for the B chain. Since the exact DNA sequence of each chain is known, they synthesize each mini-gene's DNA in an amino acid sequencing machine.
- 4 These two DNA molecules are then inserted into plasmids, small circular pieces of DNA that are more readily taken up by the host's DNA.
- 5 Manufacturers first insert the plasmids into a non-harmful type of the bacterium E. coli. They insert it next to the lacZ gene. LacZ encodes for 8-galactosidase, a gene widely used in recombinant DNA procedures because it is easy to find and cut, allowing the insulin to be readily removed so that it does not get lost in the bacterium's DNA. Next to this gene is the amino acid methionine, which starts the protein formation.
- 6 The recombinant, newly formed, plasmids are mixed up with the bacterial cells. Plasmids enter the bacteria in a process called transfection. Manufacturers can add to the cells DNA ligase, an enzyme that acts like glue to help the plasmid stick to the bacterium's DNA.
- 7 The bacteria synthesizing the insulin then undergo a fermentation process. They are grown at optimal temperatures in large tanks in manufacturing plants. The millions of bacteria replicate roughly every 20 minutes through cell mitosis, and each expresses the insulin gene.
- 8 After multiplying, the cells are taken out of the tanks and broken open to extract the DNA. One common way this is done is by first adding a mixture of lysozome that digest the outer layer of the cell wall, then adding a detergent mixture that separates the fatty cell wall membrane. The bacterium's DNA is then treated with cyanogen bromide, a reagent that splits protein chains at the methionine residues. This separates the insulin chains from the rest of the DNA.
- 9 The two chains are then mixed together and joined by disulfide bonds through the reduction-reoxidation reaction. An oxidizing agent (a material that causes oxidization or the transfer of an electron) is added. The batch is then placed in a centrifuge, a mechanical device that spins quickly to separate cell components by size and density.
- 10 The DNA mixture is then purified so that only the insulin chains remain. Manufacturers can purify the mixture through several chromatography, or separation, techniques that exploit differences in the molecule's charge, size, and affinity to water. Procedures used include an ion-exchange column, reverse-phase high performance liquid chromatography, and a gel filtration chromatography column. Manufacturers can test insulin batches to ensure none of the bacteria's E. coli proteins are mixed in with the insulin. They use a marker protein that lets them detect E. coli DNA. They can then determine that the purification process removes the E. coli bacteria.
PROINSULIN PROCESS
- 11 Starting in 1986, manufacturers began to use another method to synthesize human insulin. They started with the direct precursor to the insulin gene, proinsulin. Many of the steps are the same as when producing insulin with the A and B chains, except in this method the amino acid machine synthesizes the proinsulin gene.
- 12 The sequence that codes for proinsulin is inserted into the non-pathogenic E. coli bacteria. The bacteria go through the fermentation process where it reproduces and produces proinsulin. Then the connecting sequence between the A and B chains is spliced away with an enzyme and the resulting insulin is purified.
- 13 At the end of the manufacturing process ingredients are added to insulin to prevent bacteria and help maintain a neutral balance between acids and bases. Ingredients are also added to intermediate and long-acting insulin to produce the desired duration type of insulin. This is the traditional method of producing longer-acting insulin. Manufacturers add ingredients to the purified insulin that prolong their actions, such as zinc oxide. These additives delay absorption in the body. Additives vary among different brands of the same type of insulin.
Analog insulin
In the mid 1990s, researchers began to improve the way human insulin works in the body by changing its amino acid sequence and creating an analog, a chemical substance that mimics another substance well enough that it fools the cell. Analog insulin clumps less and disperses more readily into the blood, allowing the insulin to start working in the body minutes after an injection. There are several different analog insulin. Humulin insulin does not have strong bonds with other insulin and thus, is absorbed quickly. Another insulin analog, called Glargine, changes the chemical structure of the protein to make it have a relatively constant release over 24 hours with no pronounced peaks.
Instead of synthesizing the exact DNA sequence for insulin, manufacturers synthesize an insulin gene where the sequence is slightly altered. The change causes the resulting
Quality Control
After synthesizing the human insulin, the structure and purity of the insulin batches are tested through several different methods. High performance liquid chromatography is used to determine if there are any impurities in the insulin. Other separation techniques, such as X-ray crystallography, gel filtration, and amino acid sequencing, are also performed. Manufacturers also test the vial's packaging to ensure it is sealed properly.
Manufacturing for human insulin must comply with National Institutes of Health procedures for large-scale operations. The United States Food and Drug Administration must approve all manufactured insulin.
The Future
The future of insulin holds many possibilities. Since insulin was first synthesized, diabetics needed to regularly inject the liquid insulin with a syringe directly into their bloodstream. This allows the insulin to enter the blood immediately. For many years it was the only way known to move the intact insulin protein into the body. In the 1990s, researchers began to make inroads in synthesizing various devices and forms of insulin that diabetics can use in an alternate drug delivery system.
Manufacturers are currently producing several relatively new drug delivery devices. Insulin pens look like a writing pen. A cartridge holds the insulin and the tip is the needle. The user set a dose, inserts the needle into the skin, and presses a button to inject the insulin. With pens there is no need to use a vial of insulin. However, pens require inserting separate tips before each injection. Another downside is that the pen does not allow users to mix insulin types, and not all insulin is available.
For people who hate needles an alternate to the pen is the jet-injector. Looking similar to the pens, jet injectors use pressure to propel a tiny stream of insulin through the skin. These devices are not as widely used as the pen, and they can cause bruising at the input point.
The insulin pump allows a controlled release in the body. This is a computerized pump, about the size of a beeper, that diabetics can wear on their belt or in their pocket. The pump has a small flexible tube that is inserted just under the surface of the diabetic's skin. The diabetic sets the pump to deliver a steady, measured dose of insulin throughout the day, increasing the amount right before eating. This mimics the body's normal release of insulin. Manufacturers have produced insulin pumps since the 1980s but advances in the late 1990s and early twenty-first century have made them increasingly easier to use and more popular. Researchers are exploring the possibility of implantable insulin pumps. Diabetics would control these devices through an external remote control.
Researchers are exploring other drug-delivery options. Ingesting insulin through pills is one possibility. The challenge with edible insulin is that the stomach's high acidic environment destroys the protein before it can move into the blood. Researchers are working on coating insulin with plastic the width of a few human hairs. The coverings would protect the drugs from the stomach's acid.
In 2001 promising tests are occurring on inhaled insulin devices and manufacturers could begin producing the products within the next few years. Since insulin is a relatively large protein, it does not permeate into the lungs. Researchers of inhaled insulin are working to create insulin particles that are small enough to reach the deep lung. The particles can then pass into the bloodstream. Researchers are testing several inhalation devices much like that of an asthma inhaler.
Another form of aerosol device undergoing tests will administer insulin to the inner cheek. Known as buccal (cheek) insulin, diabetics will spray the insulin onto the inside of their cheek. It is then absorbed through the inner cheek wall.
Insulin patches are another drug delivery system in development. Patches would release insulin continuously into the bloodstream. Users would pull a tab on the patch to release more insulin before meals. The challenge is finding a way to have insulin pass through the skin. Ultrasound is one method researchers are investigating. These low frequency sound waves could change the skin's permeability and allow insulin to pass.
Other research has the potential to discontinue the need for manufacturers to synthesize insulin. Researchers are working on creating the cells that produce insulin in the laboratory. The thought is that physicians can someday replace the non-working pancreas cells with insulin-producing cells. Another hope for diabetics is gene therapy. Scientists are working on correcting the insulin gene's mutation so that diabetics would be able to produce insulin on their own.
Where to Learn More
Books
Clark, David P, and Lonnie D. Russell. Molecular Biology Made Simple and Fun. 2nd ed. Vienna, IL: Cache River Press, 2000.
Considine, Douglas M., ed. Van Nostrand's Scientific Encyclopedia. 8th ed. New York: International Thomson Publishing Inc., 1995.
Periodicals
Dinsmoor, Robert S. "Insulin: A Never-ending Evolution." Countdown (Spring 2001).
Other
Diabetes Digest Web Page. 15 November 2001. < http://www.diabetesdigest.com >.
Discovery of Insulin Web Page. 16 November 2001. < http://web.idirect.com/~discover >.
Eli Lilly Corporation. Humulin and Humalog Development. CD-ROM, 2001.
Eli Lilly Diabetes Web Page. 16 November 2001. < http://www.lillydiabetes.com >.
Novo Nordisk Diabetes Web Page. 15 November 2001. < http://www.novonet.co.nz >.
M. Rae Nelson
K. Monsen
These insulins have had a history of use of over 70 years and are unlike modern
synthetic insulins in their delivery and side effects. There have been reports of
reactions to these more natural insulins when used over long periods of time and more likely when in higher doses, such as allergy and lumps to the injected area and a resistance build up to it over a long period of time. However they are far less violent in their delivery so require the patient to stick to a more natural diet, such as low carbohydrate, high vegetable intake and nuts and fruit in moderation and little if any refined sugars and related products (maybe, the deviants that caused the problem in the first place), the more raw the better for a slower digestive pattern.